In 2021, the U.S. Food and Drug Administration (FDA) conducted a routine assignment to collect domestic multi-commodity ready-to-eat (RTE) refrigerated dips and spreads to test for Listeria monocytogenes and Salmonella spp. The agency’s goal in conducting this assignment was to determine the prevalence of these pathogens in RTE dips and spreads and remove adulterated products from the market, when possible.
The assignment, which ran from March 2021 to January 2022, found that four out of the 747 samples tested were contaminated with Salmonella or L. monocytogenes. Ready-to-eat foods can become contaminated with environmental pathogens from harborage sites in the food manufacturing environment or process. Cross-contamination from ingredients can also occur. Additionally, dips and spreads often have a pH and water activity that favor bacterial survival and outgrowth. Consumers typically eat these dips and spreads without a ‘kill step,’ such as cooking, to reduce or eliminate any pathogenic bacteria that may be present. As such, dips and spreads contaminated with L. monocytogenes or Salmonella can present a significant public health risk. Ready-to-eat dips such as hummus and cheese spreads have been associated with multiple recalls over the past few years. The FDA’s assignment was initiated due to five recalls of hummus products and six recalls of multi-commodity dips found to be contaminated with L. monocytogenes or Salmonella from FY2017 through FY2020.
During the sampling assignment, the FDA identified Salmonella Havana in a sample of hummus from a retail establishment in California. The FDA notified the manufacturer of the hummus and a recall was initiated. The FDA conducted a follow-up inspection at the manufacturer of the contaminated product to evaluate the firm’s manufacturing operations and collect environmental samples. The FDA found that the firm did not establish and implement adequate written procedures for monitoring sanitation control procedures (i.e., cleanliness of food-contact surfaces). The environmental samples collected at the follow-up inspection did not yield any positive pathogens, however, the agency still issued a warning letter after the follow-up inspection. After taking corrective actions, the firm was unable to identify a root cause for the Salmonella Havana contamination in the hummus.
The FDA identified L. monocytogenes in three samples from a retail establishment in Colorado and the agency notified the firm where the positive samples were manufactured. All three of the samples contaminated with L. monocytogenes were produced by the same manufacturer. Upon notification of the contaminated samples, the firm recalled and destroyed the products associated with the positive findings. The agency issued a Consumer Advisory in conjunction with the firm’s recall.
The agency conducted a follow-up inspection at the manufacturer of the three contaminated products. The follow-up inspection sought to evaluate the firm’s manufacturing operations and collect environmental samples to determine potential sources and routes of contamination. The FDA found L. monocytogenes in 23 swabs collected within the production environment, including samples collected from food contact surfaces. WGS analysis was conducted on the L. monocytogenes isolates obtained from both the retail product samples and the inspection environmental samples. The isolates were a genomic match to each other.
The FDA’s follow-up inspection determined the firm’s employees were not trained in food safety or hygiene practices and found multiple instances of deficient sanitation practices related to equipment, utensils, and employees. The agency determined that the firm did not respond appropriately to the positive samples identified in the sampling assignment and had not addressed objectionable conditions observed by the FDA during a previous 2020 inspection. The firm went out of business at the end of June 2021.
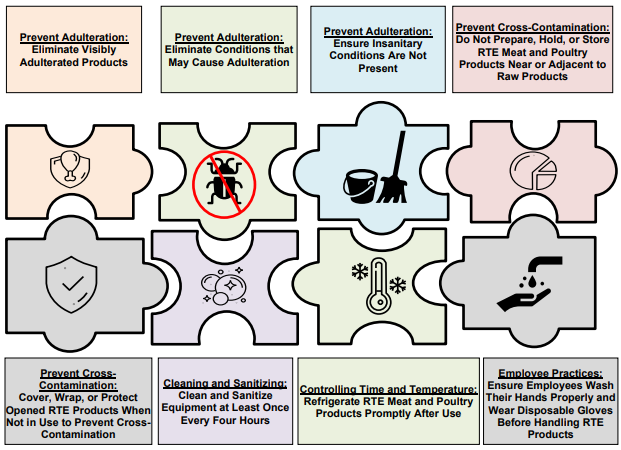
Underscored in the FDA’s report is the necessity for manufacturing firms to have good manufacturing practices in place, as well as a proper environmental monitoring program and adequate training for its staff as part of their standard business operating procedures.
Deibel Laboratories has years of experience in conducting shelf-life studies, formulation reviews, and validations for refrigerated dips and spreads. Our staff of industry experts is ready to assist you in assuring that your manufacturing processes and facilities comply with the stringent food safety standards required by the FDA and USDA. With a complete suite of accredited pathogen testing methods and the testing capacity of 15 laboratories strategically placed throughout North America, Deibel Laboratories is your one-stop for assuring food safety compliance.
Contact us at Sales@DeibelLabs.com for more information about a laboratory near you. Visit us at https://deibellabs.com/services/deibel-guardianemp/ to learn more about our environmental monitoring program, Deibel GuardianEMPsm. Link to FDA findings: FY21-22 Sample Collection and Analysis of Domestic Refrigerated RTE Dips and Spreads | FDA