The USDA Food Safety and Inspection Service (FSIS) recently published a guidance document that provides recommendations that retailers can use in delicatessens to control L. monocytogenes contamination of ready-to-eat (RTE) meat and poultry products. This 2023 document is a revised version of the 2015 FSIS Best Practices Guidance for Controlling Listeria monocytogenes (Lm) in Retail Delicatessens. It reflects the FSIS’s current thinking on this topic and is not a regulation. While focused on small firms, the recommendations made will be useful for all meat and poultry retail firms.
Recommendations are based on the joint FSIS, Food and Drug Administration (FDA), and Centers for Disease Control and Prevention (CDC) 2013 Listeria monocytogenes in Retail Delicatessens Interagency Risk Assessment. The assessment summarizes the risks posed by L. monocytogenes from the consumption of RTE foods commonly prepared and sold in retail delis.
The guidance addresses:
- Actions retailers can take in the delicatessen (deli) area to decrease the potential for
L. monocytogenes growth and cross-contamination. - Steps retailers can take to help ensure that deli products are maintained under sanitary conditions
that do not allow L. monocytogenes adulteration of the product. - Information from the U.S. Food and Drug Administration’s (FDA) Food Code, scientific literature,
other guidance documents, and lessons learned from meat and poultry establishments that
retailers can use to control L. monocytogenes. - Helpful tools that retail firms can use to identify potential gaps in current best practice procedures.
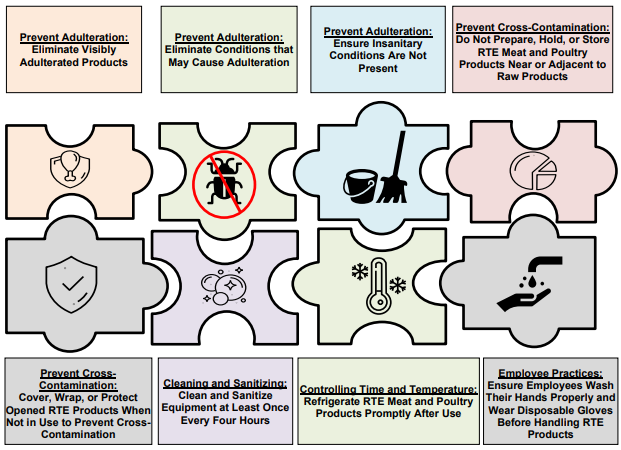
- The eight most important recommendations for retail deli operations.
Retailers can use the best practices identified in this guidance to help ensure that RTE meat and poultry products in the deli area are handled under sanitary conditions and are not adulterated, as defined in the Federal Meat Inspection Act (FMIA) and Poultry Products Inspection Act (PPIA).
While these practices are designed to control L. monocytogenes specifically, they also may help control other foodborne pathogens that may be introduced into the retail deli environment and other facilities where consumers take possession of food. No single action or practice will control L. monocytogenes contamination. Therefore, by following the best practices in this guidance and the Food Code, retailers can help ensure that RTE products are not adulterated with L. monocytogenes, and that the potential for listeriosis is decreased.
A Deli Self-Assessment Tool is provided for deli operators in Appendix A of this guidance to help them identify the best practices they are using and to assess whether they need to adopt others.
A Comprehensive Approach: The Eight Most Important Retail Deli Recommendations to Prevent
Conditions That May Cause Adulteration (Source: USDA FSIS