A symposium at IAFP 2023 (S36), “Establishing Microbiological Performance Standards for Food Safety” discussed how industry establishes performance standards for a broad category of food then validates them to meet regulatory requirements. Performance standards are the specific pathogen reduction levels that must be attained during processing to ensure that proper food safety has been achieved. These can be established based on current available scientific literature, scientific studies performed by the company, regulatory requirements, or by using
risk-based pathogen modeling. Validation then demonstrates that the performance standard can be met during routine processing of food.
Stephanie Nguyen, Principal Microbiologist at ConAgra Foods, discussed considerations for designing performance standards for ready-to-eat (RTE) meat and plant proteins, canned foods, and frozen vegetables. For cell cultured meat, FDA regulates the cell collection, banking, growth and differentiation of the cells, then oversight is transitioned to USDA-FSIS at the harvesting stage. These products need to meet USDA pathogen log reductions set forth for meat products from which the original cells originated from (i.e. FSIS Appendix A). Plant based protein products
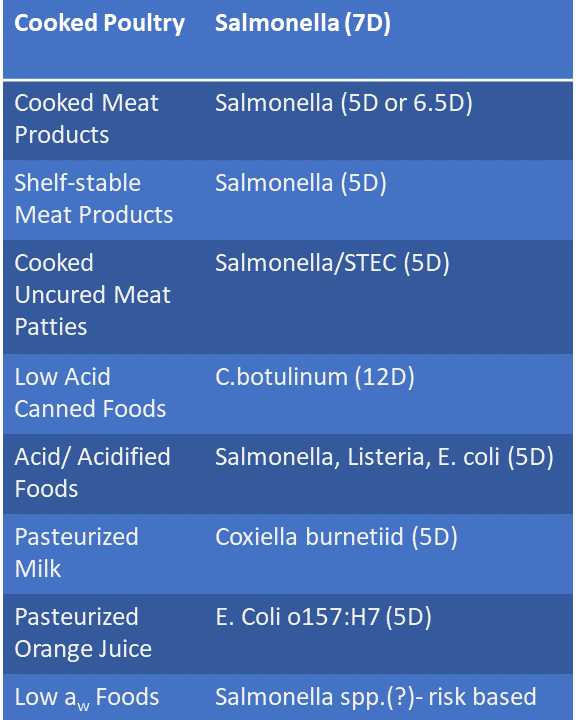
should attain a minimum 5-log pathogen reduction, the performance standard generally considered acceptable for FDA regulated products in the absence of other readily available performance standards. Performance standards for low acid canned foods are outlined in 21 CFR Part 113 and consist of applying a 12-log reduction in spores while acidified canned food guidance is outlined in 21 CFR part 114. Frozen vegetables are unique in that they are non RTE, therefore validated cooking instructions can provide the consumer necessary requirements to attain a 5-log reduction in pathogen hazards.
Rico Suhalim, Process Authority at PepsiCo, discussed considerations for designing performance standards for juices, sweet and salty snacks, including low-water activity (aw) foods. For acidified juices, HACCP guidance is available from FDA. Thermal processing to meet a minimum 5-log pathogen reduction for acidified foods pH 4.1–4.6 is described by Breidt et al., 2014. For low aw snacks, challenge studies and process validations are generally conducted to meet a 5-log pathogen reduction. It is important to control microbial load with incoming raw ingredients and understand that thermal processes designed for one food matrix may not be appropriate for another (i.e. kibble ≠ meat).
Aaron Uesugi, Principal Scientist at Mondelez, discussed considerations for designing performance standards for bakery and confectionery products. Processors should consider whether they are baking or drying to assess microbial end product risks appropriately. Bakery products can generally be validated for a 5-log pathogen reduction. However low moisture raw ingredients placed on top of the raw dough, such as sesame seeds, should be assessed to make sure they also achieve a 5-log pathogen reduction during the baking process. It is also important to control microbial hazards that may be introduced post process. For example, chocolate can’t be heated to reduce Salmonella risk, roasting the cocoa bean is the only 5- log pathogen kill step, thus sanitation preventive controls verified by an environmental monitoring program are critical to the production of safe product.
A recording of the symposium is available from IAFP and a 2022 publication by one of the presenters provides more information on this topic. Deibel Laboratories has process authorities available to design and conduct challenge studies and validations to meet regulatory safety requirements for your food products.