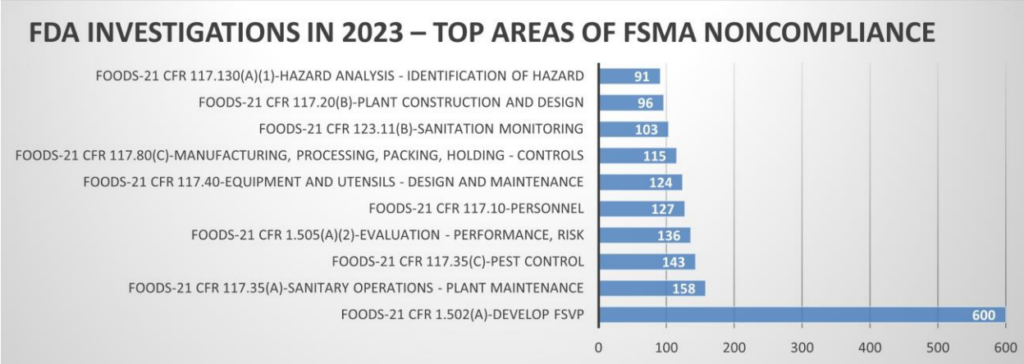
A recall and FDA public health alert has been issued for apple cinnamon fruit puree pouches after reports of acute lead exposure in children led to an investigation and finding of extremely high concentrations of lead in multiple product lots tested by health officials in North Carolina. An FDA investigation into how such high levels of lead contaminated the product is ongoing. However, since only products containing cinnamon had
elevated levels of lead, FDA’s leading hypothesis is that cinnamon used in these recalled pouches is the likely source of contamination. FDA is
currently trying to collect and test samples of the cinnamon sourced from Ecuador that was used in the recalled products as well as screen incoming shipments of cinnamon from multiple countries for lead contamination.
As of November 22, 2023, there are 52 reports of illness in children ages 1 to 4 years, with blood lead levels (BLLs) ranging from 4 to 29 micrograms per deciliter (µg/dL), potentially linked to recalled product. The FDA has found levels of lead in the recalled product as high as 2.18 ppm, 200 times higher than proposed action levels for similar products intended for babies and young children. Acute lead exposures are uncommon and have historically resulted from exposures to high concentrations of lead containing folk remedies, paint chips, or foreign bodies. Children exposed to large amounts of lead can develop gastrointestinal, hematological, and neurological effects. Symptoms include anemia, abdominal pain, weakness, and severe neurological sequelae (e.g., seizures, encephalopathy, and coma), which may result in brain damage. Elevated BLLs in children above 45 µg/dL may require hospital admission for monitoring and chelation therapy.
Lead is a naturally occurring heavy metal but also a man-made pollutant. According to the FDA and CDC, lead exposures related to food can involve:
- water sourced from lead pipes, faucets, and plumbing fixtures;
- fruits, vegetables, or grains grown in areas with lead containing soil, air, and water that resulted
naturally or by contamination from human-activities such as pollution; - animals raised in high lead environments or that consumed lead contaminated feed;
- leeching from lead contaminated food contact surfaces;
- economically motivated adulteration – for example, lead-based dyes used to impact color have
been found in spices such as chili powder, turmeric, and cumin.
While no level of lead exposure is safe for children, minimizing exposure from food is the focus of FDA’s Closer to Zero action plan. Draft guidance was issued in January 2023 proposing action levels for lead in food intended for babies and young children. It establishes the following action levels:
- 10 parts per billion (ppb) for fruits, vegetables (excluding single-ingredient root vegetables),
mixtures (including grain and meat-based mixtures), yogurts, custards/puddings, and singleingredient meats; - 20 ppb for root vegetables (single ingredient); and
- 20 ppb for dry infant cereals.